Products You Can Trust
Goodness Care Develops High-Quality Health Products: Supplements, Medical Devices, and Cosmetics
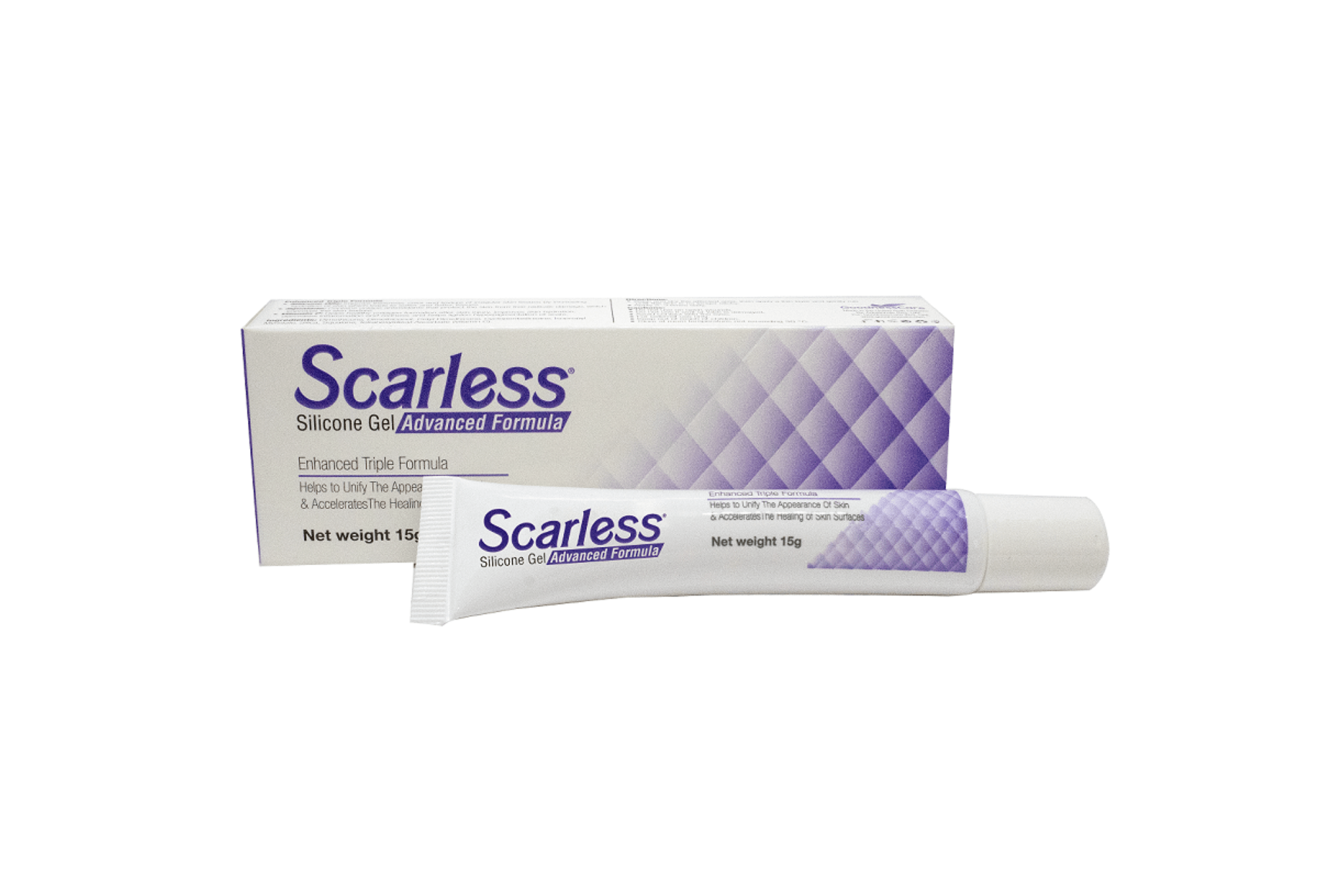
Advanced Unique Formula of Silicone Oils, Squalane, and Vitamin C. Helps Reduce Scar Thickness, Redness, and Discoloration. Works for Old and New Scars.
Vaginal Gel for Moisturizing & Restoration. Reduces Vaginal Dryness & Itching. Restores Structure & Elasticity of Vaginal Tissues and Provides Firming and Tightening effect.
Unique Natural Formula Gel for Men to Extend Intimacy, Satisfaction, and Enjoyment. Safe, Does Not Include Lidocaine or Benzocaine, and Does Not Cause Numbing.
13 Powerful Digestive Enzymes Supplementation to Support Healthy Digestion, Reduce IBS Symptoms, Crohn’s, and Celiac Disease Symptoms, Supplement Pancreatic Enzymes, and Increase Nutrient Absorption.
Vitamin D3 and Omega 3 Supplement to Support Healthy Growth Of Children’s Brains, Bones, and Immune Systems.